
COMMON MILK ADULTERATIONS AND THEIR DETECTING TECHNIQUES
Pashu Sandesh, 01 September 2020
DR.PRIYA KALLA
Food adulteration is a global concern. This is one of the most common phenomena that have been overlooked in many countries. Milk adulterants can pose serious health hazards leading to fatal diseases. The motivation for food fraud is economic, but the impact is a real public health concern. Food adulteration is a serious issue which has needed to investigate as potential food safety and public health concern in recent years.
Food fraud is defined as “the fraudulent, intentional substitution or addition of a substance in a product for the purpose of increasing the apparent value of the product or reducing the cost of its production,” and can open encompass effects public safety through the unknown addition of allergens, toxins, and hygienic risks.
Nowadays it is very common to hear or read news about the food items being adulterated and such products are being openly sold out and are consumed by people, which cause various health hazards.
Milk: Milk is defined as the clean, whole, fresh, lacteal secretion obtained by the complete milking of one or more healthy milk animals excluding that obtained within (15) days before or (5) days after the calving or such period as may be necessary to provide the milk practically colostrums free and containing the minimum prescribed percentage of sold not fat and milk fat. Milk is considered to be the ‘ideal food’ because of its abundant nutrients required by both infants and adults. It is one of the best sources for protein, fat, carbohydrate, vitamin and minerals. Milk contains 87% water, 3.3% protein, 3.9% fats, 5% lactose and 0.7% ash. Unfortunately, milk is being very easily adulterated throughout the world. Possible reasons behind it may include- demand and supply gap, perishable nature of milk, low purchasing capability of the customer and lack of suitable detection tests.
Milk is adulterated either intentionally or accidentally during the production and processing of milk. There are many potential adulterants in liquid milk, such as neutralizers, salt, sugar, water, or solid content
Methods for Detection of common adulterants in Milk
Tests which have done at home:
|
Adulterant |
Method of detection |
|
Water |
The presence of water can be detected by putting a drop of milk on a polished slanting surface. The drop of pure milk flows slowly leaving a white trail behind it, whereas milk adulterated with water will flow immediately without leaving a mark. |
|
Starch |
Add a few drops of tincture of Iodine or Iodine solution. Formation of blue colour indicates the presence of starch. |
|
Urea |
Take a teaspoon of milk in a test tube. Add half teaspoon of soybean or arhar powder, Mix up the contents thoroughly by shaking the test tube. After 5 minutes, dip a red litmus paper in it. Remove the paper after half a minute. A change in colour from red to blue indicates the presence of urea in the milk. |
|
Detergent |
Shake 5-10 ml of sample with an equal amount of water. Lather indicates the presence of detergent |
|
Synthetic milk |
Synthetic milk has bitter after taste, gives a soapy feeling on rubbing between the fingers and turns yellowish on heating |
|
Synthetic milk - test for protein |
The milk can be easily tested using Urease strips. Colour chart in Urease strips helps to arrive at the quantity of urea present in the milk. |
|
Test for Glucose /Invert sugar |
Take a strip of Diacetric strip and dip into the milk for 30 sec to 1 min. If the strip changes the colour then it shows the sample of milk contains glucose. If there is no change in the colour of the strip, then glucose is absent
|
Tests which have to be done in Laboratory
|
Adulterant |
Method of detection |
|
|
|
|
Vanaspati |
Take 3 ml of milk in a test tube. Add 10 drops of Hydrochloric Acid. Mix one teaspoonful of sugar. After 5 minutes, examine the mixture. The red colouration indicates the presence of vanaspati in the milk |
|
||
|
formalin |
Take 10ml of milk in a test tube and add 5 ml of concentrated Sulphuric acid from the sides of the wall without shaking. If a violet or blue ring appears it shows the presence of formalin. |
|
|
|
|
Ammonium Sulphate |
•Take 5 ml of hot milk in a test tube. Add a suitable acid, eg., Citric Acid. The whey obtained is separated and filtered. Take the whey in another test tube and add 0.5 ml of 5% Barium Chloride. The appearance of a precipitate indicates the presence of Ammonium Sulphate. |
|
|
|
|
|
|
|
|
|
|
Hydrogen Peroxide |
• Take 5 ml milk in a test tube. Add 3 drops of Paraphenylene Diamine and shake well. Change in colour of the milk to blue confirms that the milk is adulterated with Hydrogen Peroxide, |
|
|
|
|
Sugar |
Take 3 ml of milk in a test tube. Add 2 ml of the hydrochloric acid. Heat the test tube after adding 50 mg of resorcinol, The red colouration indicates the use of sugar in the milk. |
|
|
|
|
Sodium bi-carobonate / Neutralizer |
Take 3 ml of milk in a test tube and add 5 ml of rectified spirit to it. Then add 4 drops of rosalic acid solution. The appearance of red/rosy colouration indicates the presence of Sodium bicarbonate in the milk. |
|
|
|
|
Boric acid |
Take 3 ml of milk in a test tube. Add 20 drops of hydrochloric acid and shake the test tube or mix up the contents thoroughly. Dip a yellow paper- strip, and remove the same after 1 minute. A change in colour from yellow to red, followed by the change from red to green, by addition of one drop of ammonia solution, indicates that the boric acid is present in milk |
|
|
|
|
Removal of Fat |
The Lactometer reading will go above 26 |
|
|
|
|
|
|
|
|
|
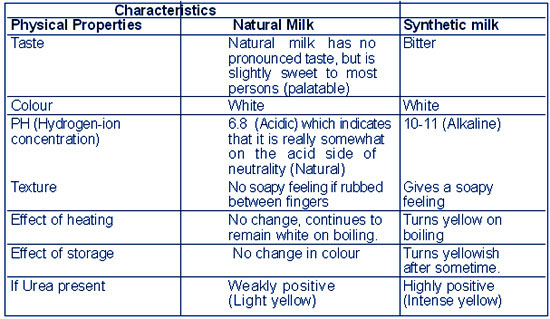
PHYSIO-CHEMICAL DIFFERENCE IN
SYNTHETIC MILK &NATURAL MILK
The adulteration of milk and milk products is punishable under I.P.C. Section 272.
DR.PRIYA KALLA
(Teaching Associate),
College of Veterinary & Animal Science, RAJUVAS, BIKANER










































































